Strain counterstrain is an osteopathic manipulative technique about which research is only recently emerging. This master class reviews the evidence investigating proposed physiologic mechanisms and clinical effects of strain counterstrain. Clinical application guidelines are presented with specific treat- ments for key clinical scenarios.
1. Introduction
Strain Counterstrain (SCS) is the fourth most commonly used osteopathic manipulative technique following soft tissue tech- niques, high velocity low amplitude thrust, and muscle energy technique (Johnson and Kurtz, 2003). Also known as positional release, SCS is a passive positional technique aimed at relieving musculoskeletal pain and dysfunction through indirect manual manipulation (d’Amborgio and Roth, 1997).
Accurate palpation of diagnostic tender-points (TP) is central to SCS. TPs have been described as tender upon palpation, small (<1 cm), round, edematous, and found in muscle, tendon, ligament, or fascial tissues (Jones, 1995), unlike trigger points which are found within taut musculotendinous tissue bands (Simons et al., 1999; Lewis et al., 2008). Symptomatic patients have lower elec- trical detection and pain thresholds for TPs than controls (Lewis et al., 2010a).
SCS treatment begins by identifying diagnostic TPs related to musculoskeletal dysfunction. Once a tender TP is identified, the practitioner manually monitors the TP while positioning the patient that relieves palpation tenderness. This position-of-comfort is typically obtained by shortening tissues around the TP. The practitioner need not maintain palpation pressure while support- ing the patient passively in the position-of-comfort, but may maintain gentle touch on the TP to assure accurate palpation afterward. Release of the TP occurs after 90-s, after which the practitioner slowly returns the patient to normal resting position (Jones, 1995; d’Amborgio and Roth, 1997; Chaitow, 2007).
Since the osteopathic doctor Lawrence Jones first reported using SCS (Jones, 1964), over 200 TPs have been identified. Descriptive cases documented SCS applications for foot (Jones, 1973), knee (Haman, 1994; Pedowitz, 2005), lower back (Ramirez et al., 1989; Cislo et al., 1991; Lewis and Flynn, 2001), shoulder (Jacobson et al., 1990), and myofascial disorders (Dardzinski et al., 2000). Some studies combined SCS with other treatments for disorders including complex regional pain syndrome (Collins, 2007), cervicothoracic pain (Walko and Janouschek, 1994; Nagrale et al., 2010), lateral epicondylalgia (Benjamin et al., 1999), and cavus foot (Wong et al., 2010). Others suggest SCS for abdominal pain (Alexander, 1999), pancreatitis (Radjieski et al., 1998), and other visceral dysfunctions (Giammatteo and Weiselfish, 1997) and medical diagnoses (Schwartz, 1986; Licciardone, 2004).
This master class 1) discusses proposed physiologic mechanisms in the context of relevant literature, 2) reviews evidence supporting clinical use, 3) presents clinical application guidelines, and 4) describes SCS treatments for several clinical scenarios.
2. Proposed physiologic mechanisms
The mechanisms explaining the effects of SCS reported in clin- ical practice remain largely theoretical. Suggested factors in SCS intervention include aberrant neuromuscular activity mediated by muscle spindles and local circulation or inflammatory reactions influenced by the sympathetic nervous system (Chaitow, 2007).
Aberrant neuromuscular activity between muscle agonist and antagonist, known as the Proprioceptive Theory, is the most common explanation for the effects of SCS. According to the Proprioceptive Theory rapid stretching injury stimulates muscle spindles causing reflexive agonist muscle contraction that resists further stretching. However, a reflexive counter-contraction resulting from pain- induced withdrawal quickly reverses the aggravating movement thereby exciting antagonist muscle spindles. The resulting neuro- muscular imbalance, perpetuated by opposing muscle spasms each unable to release due to ongoing muscle spindle excitation (Korr, 1975), can affect myofascial mobility and force transmission around neighboring joints and muscles (Kreulen et al., 2003; Huijing and Baar, 2008). Underlying muscle imbalance can persist long after the strain heals (Korr, 1975; Goering, 1995) with lasting motor impair- ment evident long after pain symptoms subside (Sterling et al., 2003). The Proprioceptive Theory is based on neurophysiologic regulation of muscle spindle activity that increases spindle activity and reflexive muscle contraction upon lengthening and decreases spindle discharge and reflexive contraction upon shortening (Korr, 1975). By passively shortening the dysfunctional agonist muscle long enough, SCS allows normal muscle spindle activity to return. Once agonist muscle spindle activity is reset, antagonist muscle spindle activity can also return to resting state, relieving aberrant neuromuscular activity and restoring normal function (Jones, 1995).
Few studies have attempted to validate the Proprioceptive Theory. One randomized controlled study demonstrated increased strength after SCS treatment for hip TPs in opposing muscle groups. Secondary analysis demonstrated no correlation between the increased strength of agonist and antagonist muscles (Wong and Schauer-Alvarez, 2004). Direct assessments of the Proprioceptive Theory produced conflicting results. A caseecontrol study of patients with Achilles tendonitis found that Achilles tendon stretch reflex amplitudes reduced after SCS, but the H-reflexdwhich bypasses the muscle spindlesdremained unchanged, suggesting that SCS might affect the stretch reflex by altering muscle spindle activity (Howell et al., 2006). However, foot and ankle SCS treat- ments did not change either reflex amplitudes in a randomized controlled crossover study of patients with plantar fasciitis despite reduced pain and increased plantarflexion reflex torque (Wynn et al., 2006). Neither study standardized Achilles tendon SCS treatment, which might account for observed differences and complicates interpretation of the conflicting results.
The suggestion that SCS affects local circulation was partly based on a cadaver study in which a zone of relative rotator cuff tendon avascularity was found to be position-dependent and relieved once the tendons were placed in a shortened position (Rathbun and Macnab, 1970). Through positioning, SCS may increase local circu- lation hastening nutrient supply and metabolic waste removal in living tissue (Jacobson et al., 1990). Improved circulation would reduce swelling that otherwise inhibits muscle function (McNair et al., 1996) and reverse ischemia that can manifest as painful TPs or sustain dysfunction (Goering, 1995; Mense and Simons, 2001).
One laboratory study attempted to validate the circulatory effects of SCS. Human fibroblasts stretched beyond resting length secreted more pro-inflammatory interleukins and decreased cell proliferation compared to resting cells (Meltzer and Standley, 2007). Once returned to resting length for a minute of positional release, the fibroblasts secreted lower levels of pro-inflammatory interleukin IL-6 and significantly increased cell proliferation compared to after stretch. Multiple interleukin functions, related growth factors, and in-vitro testing make firm conclusions difficult. Nevertheless, decreased IL-6 levels, important for mediating inflammatory healing after acute injury (Kopf et al., 1994), suggest SCS may affect local circulation (Meltzer and Standley, 2007). Clinically, Achilles tendonitis patients reported decreased swelling after SCS (Howell et al., 2006) but research is needed to understand potential circulatory effects of SCS.
SCS may affect the protective ligamento-muscular reflex (Chaitow, 2009) through which ligamentous strain inhibits muscle contractions that increase strain, or stimulates muscles that reduce strain, to protect the ligament (Krogsgaard et al., 2002; Solomonow, 2009). For instance, anterior cruciate ligament strain inhibits quadriceps and stimulates hamstring contractions to reduce ante- rior tibial distraction (Dyhre-Poulsen and Krogsgaard, 2000). Ligamentous reflex activation also elicits regional muscle responses that indirectly influence joints (Solomonow and Lewis, 2002). Research is needed to explore whether SCS may alter the protective ligamento-muscular reflex and thus reduce dysfunction by short- ening joint ligaments or synergistic muscles (Chaitow, 2009).
How SCS affects the body remains unclear, but research exam- ining the theoretical underpinnings of SCS has laid the groundwork for future research. Research has recently been emerging to support clinical use of SCS.
3. Evidence to support clinical use
Several studies investigating SCS report decreased pain or palpation tenderness. The first randomized control study had subjects with hip TPs receive SCS, exercise, or SCS and exercise. While treatment decreased pain for all subjects, those receiving SCS demonstrated significantly larger decreases than those receiving exercise alone (Wong and Schauer, 2004). Groups receiving SCS had medium effect sizes (d 1⁄4 0.49e0.76) but conclusions must be drawn with caution because the study lacked blinding, sham positioning, standardized palpation pressure, or patients with clinical pathology.
Several studies suggest SCS may reduce upper trapezius pain. Subjects with self-reported upper trapezius stiffness and pain were randomly assigned to receive SCS or sham positioning treatment in a blinded study. Both sham (d 1⁄4 0.40) and SCS (d 1⁄4 0.71) imme- diately reduced palpation pain, but 24 h after treatment no differ- ences existed between groups (Perreault et al., 2009). In another blinded randomized controlled study, patients with mechanical neck pain diagnosis received SCS, SCS with massage, or simple rest. Pain significantly decreased upon palpation with standardized pressure for both groups receiving SCS (d > 1.0), while the control group experienced no change (Meseguer et al., 2006). Neck pain patients receiving muscle energy technique or SCS with muscle energy technique and ischemic compression had similar results in a similarly designed study (Nagrale et al., 2010). Overall, people diagnosed with neck pain appear to benefit from SCS more than healthy people with TPs, but more blinded studies with sham treatments including touch are needed.
For trapezius and masseter trigger points, SCS appears effective in reducing palpation pain. SCS treatment for latent masseter trigger points, diagnosed using myofascial pain diagnostic criteria (Simons et al., 1999), also reduced pain in a blinded randomized control study of subjects receiving SCS, massage, or no treatment. Subjects receiving SCS and massage had significantly decreased pain pres- sure thresholds (d > 1.0) and palpation pain (d > 0.50), while pain increased after no treatment (d < 0.20) (Ibanez-Garcia et al., 2009). Effectiveness of SCS treatment for trigger points cannot be gener- alized, however, as not all TPs are located within muscles.
Furthermore, while SCS can reduce TP tenderness, clinical pain and disability may not be improved (Lewis et al., 2010b) and studies reporting follow-up measures found only some pain relief preserved after 1 (Howell et al., 2006) to 2e4 weeks (Wong and Schauer, 2004).
Effect of SCS on range-of-motion was first investigated in a blinded randomized crossover study of hamstring length measured with straight-leg-raise, which reported no change after SCS or sham positioning (Birmingham et al., 2004). A later blinded randomized controlled study, found both SCS and no treatment had no effect on jaw opening, while contract-relax stretching increased range-of-motion (Blanco et al., 2006). In a similarly designed study by the same researchers, SCS or massage increased jaw opening range-of-motion (d > 1.0) more than no treatment (Ibanez-Garcia et al., 2009). These well-designed studies provide conflicted support for using SCS to improve range-of-motion.
Two randomized control studies with same lead author exam- ined the effect of SCS on strength. The first found 41e73% increases in hip strengthdmeasured with handheld dynamometerdafter SCS with or without exercise (d 1⁄4 0.64e1.16) compared to exercise alone (Wong and Schauer-Alvarez, 2004). The second, using assessor- and subject-blinding, found that subjects with painful elbow TPs had significantly increased forearm strength of 8e12% (d 1⁄4 0.76e0.83) 1-week after SCS treatment compared to sham manual positioning (Wong et al., in press). Both studies had medium to large treatment effect sizes; differences in percent strength changes between studies may be attributed to different numbers of treatments and strength assessment methods.
Overall, clinical research into SCS has only begun to emerge. While evidence suggests SCS may relieve pain in some body regions, inconsistent study designs limit the conclusions that can be drawn.
4. Clinical applications of strain counterstrain
SCS is typically used to treat orthopedic disorders involving pain, fascial tension, local edema, joint hypomobility, muscle spasm, muscle dysfunction or weakness (d’Ambrogio and Roth, 1997). Assuming the practitioner listens and responds to patient feedback without forcing treatment through patient discomfort, SCS is con- traindicated primarily when the patient cannot provide feedback or has medical conditions that generally exclude manual therapy.
SCS treatment begins with assessment of specific TPs to be monitored during and after treatment. The TPs, often named for bony landmarks, are purportedly associated with orthopedic dysfunction. For instance, Anterior-Cervical 7 (AC7) is so named for its clinical association with 7th cervical dysfunction. Some SCS practitioners use a palpation pain scale that includes the “jump sign” or sudden physical withdrawal from palpation (Simons et al., 1999), but this 3-point scale demonstrated only fair agreement (kappa 1⁄4 0.23e0.33) (Wong and Schauer, 2004). A visual analog scale is more reliable, but cumbersome in clinical practice. Alter- nately, a dichotomous (present or absent) TP assessment demon- strated moderate agreement (kappa 1⁄4 0.45) in symptomatic patients (McPartland and Goodridge, 1997). Further research is needed to establish the reliability and diagnostic validity of TP palpation assessments and it is suggested that other clinical assessments of physical impairment and functional limitation such as range-of-motion, joint mobility, strength, and functional ability accompany treatment.
To treat a specific TP, the practitioner passively moves the patient into the position-of-comfort while monitoring the TP. The position-of-comfort is the patient position in which the TP is least tender: at least 70% less tender than at assessment (Chaitow, 2007), but preferably completely non-tender. General guidelines for obtaining the position-of-comfort follow.
Shorten tissues containing the TP by bending joints around the TP. For instance, the anterior ankle TP position-of-comfort includes ankle dorsiflexion.
Shorten in the relevant cardinal planes. TPs close to midline often are relieved with shortening predominantly in one plane; TPs further from midline require three-dimensional positions. For instance, the anterior ankle TP position-of-comfort is dor- siflexion, the position-of-comfort for the lateral ankle TP,
located on the anterolateral talus, includes ankle dorsiflexion,
eversion, and external rotation.
The position-of-comfort should relieve both TP tenderness and
fascial tightness. While monitoring during positioning, TP tenderness may vary as the position-of-comfort is approached, passed, and returned to. Positioning is fine-tuned with slight motions in secondary planes.
The exact position-of-comfort varies and never causes pain at the TP or elsewhere. When a patient’s mobility is limited, the position-of-comfort is modified and never requires motion beyond the limit of patient comfort. A common error is to position the related tissue in the most shortened position.
When a line of TPs exists, such as along the spine, treating the middle TP first may produce benefit both proximal and distal.
The practitioner supports the patient in the position-of-comfort for 90 s (Jones, 1995). Practitioners have suggested times from 5 s to 3 min for positioning when combining isometric contractions or ischemic compression as in facilitated release, functional technique, and integrated neuromuscular inhibition technique. Nevertheless, the 90-s hold time remains standard even if still unvalidated (Chaitow, 2007). Different TPs may require different hold times, perhaps best dictated by reaching the desired outcome: palpable tissue ease and decreased tenderness. Intermittent TP palpation while maintaining the position-of-comfort is clinically useful for monitoring changes and assuring consistent palpation upon reassessmentdindeed, success depends on accurate and sensitive palpation. The practitioner slowly returns the patient to neutral resting position after treatment and repeats important clinical assessments.
Clinical SCS application can be central or integrated in a plan-of- care. Some suggest screening the body for TPs, then treating according to clinical priority (d’Ambrogio and Roth, 1997). When central to the plan-of-care, SCS is used to treat the most tender TPs first, concentrated areas of TPs before diffuse areas, and proximal TPs before distal (Kusunose, 1995).
In an integrated approach, TP screening is just one element in a thorough assessment that includes palpation, muscle flexibility, joint mobility, range-of-motion, strength, and functional move- ment in the context of patient participation in life’s activities. Potentially relevant regions are screened for TPs, since distant segments are interrelated through the body’s kinetic chains. In the upper limb, for instance, cervicothoracic spine, rib, and shoulder girdle impairment may all contribute to symptoms and dysfunc- tion. Painful areas, key transitional zones of movement (e.g. cervi- cothoracic junction, shoulder and pelvic girdles, rearfoot), and TPs associated with impaired joint function take clinical priority.
While multiple techniques can address these priority areas, several scenarios make SCS an appropriate choice. On the initial day, SCS makes an excellent approach because treatment is both gentle and effectivedcritical for building clinician-patient trust. SCS can facilitate treatment for patients who express anxiety about experiencing pain during treatment, have low pain tolerance levels, cannot get into position for other techniques, or have dysfunctions with few treatment options. SCS is also useful for reducing local pain and preparing tissues for treatments like joint manipulation, soft tissue mobilization, stretching, or strengthening. Active treat- ment including exercise or functional training should follow SCS treatment to maintain gains and prevent passivity.
5. Strain counterstrain techniques
5.1. Anterior cervical
A common TP exists at the Anterior Cervical 7 (AC7) level, the critical cervicothoracic transitional juncture (Jones, 1995). Located on the superior surface of the medial clavicle 2e3 cm from the sternoclavicular joint, AC7 is associated with sternocleidomastoid muscle tightness and sternoclavicular joint inferior glide hypo- mobility. AC7 dysfunction can influence clinical scenarios involving shoulder impingement, rotator cuff syndrome, tendonitis, and overhead dysfunction; cervical or shoulder girdle malposition and dysfunction like mechanical neck pain, myofascial pain, and postural dysfunctions. SCS for AC7 can improve shoulder range-of- motion, strength, overhead functional ability, and pain.
With patient resting supine, the practitioner palpates AC7 with caudal directed force near the sternocleidomastoid origin. The position-of-comfort is obtained by first rotating the head almost fully away, then raising the head into flexion with ipsilateral side- bending (Fig. 1), shortening the sternocleidomastoid. Supporting the patient’s head with a pillow on the practitioner’s thigh allows the patient to rest without tension. Other anterior cervical TPs, palpated with posterior directed force on the anterior transverse processes, have similar positions-of-comfort in level-specific cervical flexion, sidebending, and rotation: e.g. AC3 requires only gentle traction to induce C3 flexion and slight sidebending and rotation away. (Fig. 2)
5.2. Elbow-forearm
Considered the most important elbow TP, the supinator (SUP) TP (Jones, 1995) resides at a key upper limb juncture that determines hand placement for all manual activities. Located over the anterior radial head and associated with radial head mobility, SUP influ- ences elbow, forearm, wrist, and hand movements. The SUP and pronator (PRO) TPs contribute to clinical scenarios involving distal upper limb dysfunction like epicondylalgia, tendonitis, myofascial pain, and adverse neural tension. Forearm SCS can benefit distal upper limb range-of-motion, strength, functional ability, and pain (Wong et al., in press).
For SUP and PRO treatment, the patient rests supine. The prac- titioner palpates SUP with posterior directed force directly over the radial head 1e2 cm distal to the elbow. The position-of-comfort is obtained with full, but gentle, elbow extension, forearm supination, then fine-tuning with slight elbow valgus. Knowing the elbow will not be forcefully extended reassures the patient. (Fig. 3) PRO is palpated with posterior directed force on the anteromedial fore- arm, 1e2 cm distal to the elbow. The position-of-comfort is ob- tained with w90 elbow flexion, forearm pronation, then slight shoulder internal rotation for fine-tuning. (Fig. 4)
5.3. Sacroiliac joint
TPs located at the superior (SSI) and inferior (ISI) sacroiliac joint, a key zone transferring forces between lower limb and spine, contribute to clinical scenarios like mechanical back pain, pelvic
Fig. 8. Lateral Ankle (LAN) positioning, black arrow points to LAN tender point (inset): note foot external rotation.
floor dysfunction, hip bursitis, and patellofemoral pain syndrome. SCS for the sacroiliac joint may reduce hip and lumbar range-of- motion limitations; abdominal and proximal lower limb weak- ness, lumbopelvic pain and postural dysfunctions, and related functional limitations.
With patient prone, the practitioner palpates SSI with medial directed force on the lateral aspect of the posterior superior iliac spine. The position-of-comfort is obtained with hip abduction and extension, producing anterior ilial rotation, and slight hip internal- external rotation for fine-tuning. Resting the leg on the practi- tioner’s thigh assures the patient remains relaxed (Fig. 5). The ISI TP is palpated with medial directed force perpendicular to the lateral sacral edge approximately 4 cm below the posterior superior iliac spine. The position-of-comfort is obtained by lifting the leg into extension with slight hip adduction and external rotation for fine- tuning (d’Amborgio and Roth, 1997). (Fig. 6)
5.4. Rearfoot joints
The subtalar and ankle joints form an important transitional complex affecting the entire lower extremity. Dysfunction at either joint is associated with a range of common orthopedic disorders such as ankle and foot sprains, plantar fasciitis, tendonitis, and knee pain. The lateral calcaneal (LCA) TP associated with the subtalar joint, talar TP (TAL) associated with the talocrural joint, and lateral ankle (LAN) TP associated with the anterior-talofibular ligament can affect clinical scenarios involving lower limb kinetic chain. Rearfoot SCS can improve rearfoot range-of-motion and strength; foot, ankle and knee pain; balance, gait, and functional ability (Jones, 1973; Wong et al., 2010).
LCA is palpated with middle finger applying medial directed force to the lateral calcaneal tuber. With patient sidelying on the ipsilateral leg and practitioner grasping the plantar heel, the position-of-comfort is obtained by leaning onto the calcaneus to create rearfoot eversion while the distal hand holds the anterior talus causing rotation around the subtalar joint axis (Fig. 7). Palpated in the sinus tarsi on the anterolateral corner of the talus, the LAN TP is relieved in the same position except that the practi- tioner’s distal hand rotates the foot down into significant external rotation to obtain the position-of-comfort (Fig. 8).
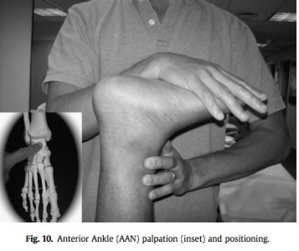
The TAL TP is palpated with posterolateral directed force on the anterior medial talar corner. With patient prone, the position-of- comfort is obtained with w90 knee flexion, ankle dorsiflexion, and subtalar joint inversion (Fig. 9). The position-of-comfort for a TP more directly anterior at the ankle is obtained the same way, but with less inversion (Fig. 10).
6. Conclusion
SCS techniques have been presented that highlight how the positioning guidelines are applied and provide the clinician tools to use at important transitional zones. The general suggestions for SCS use in common clinical scenarios may guide practitioners to successful outcomes. While evidence to support SCS has only begun to emerge, clinical applications may stimulate further controlled research into the physiologic mechanisms and clinical outcomes of SCS.
References
Alexander K. Use of strain-counterstrain as an adjunct for treatment of chronic lower abdominal pain. Physical Therapy Case Reports 1999;2(5):205e8.
Benjamin SJ, Williams DA, Kalbfleisch JH, Gorman PW, Panus PC. Normalized forces and active range of motion in unilateral radial epicondylalgia. Journal of Orthopedic and Sports Physical Therapy 1999;29:668e76.
Birmingham TB, Kramer J, Lumsden J, Obright KD, Kramer JF. Effect of a positional release therapy technique on hamstring flexibility. Physiotherapy Canada 2004; 56:165e70.
Blanco CR, de las Penas C, Xumet JE, Algaba CP, Rabadan MF, de la Quintana MC. Changes in active mouth opening following a single treatment of latent myo- fascial trigger points in the masseter muscle involving post-isometric relaxation or strain-counterstrain. Journal of Bodywork and Movement Therapies 2006; 10:197e205.
Chaitow L. Positional release techniques. 3rd ed. Philadelphia: Churchill Livingstone Elsevier; 2007.
Chaitow L. Ligaments and positional release techniques. Journal of Bodywork and Movement Therapies 2009;13:115e6.
Cislo S, Ramirez MA, Schwartz HR. Low back pain: treatment of forward and backward sacral torsions using counterstrain techniques. Journal of the Amer- ican Osteopathic Association 1991;91:255e9.
Collins CK. Physical therapy management of complex regional pain syndrome in a 14-year-old patient using strain counterstrain: a case report. Journal of Manual and Manipulative Therapy 2007;15(1):25e41.
D’Ambrogio KJ, Roth GB. Positional release therapy: assessment and treatment of musculoskeletal dysfunction. St. Louis: Mosby; 1997.
Dardzinski JA, Ostrov BE, Hamann LS. Myofascial pain unresponsive to standard treatment: successful use of a strain and counterstrain technique with physical therapy. Journal of Clinical Rheumatology 2000;6(4):169e74.
Dyhre-Poulsen P, Krogsgaard MR. Muscular reflexes elicited by electrical stimula- tion of the anterior cruciate ligament in humans. Journal of Applied Physiology 2000;89:2191e5.
Giammatteo T, Weiselfish-Giammatteo S. Integrative manual therapy for the autonomic nervous system and related disorders. Berkeley: North Atlantic Books; 1997.
Goering EK. Physical manipulation. In: Strain-counterstrain. Indianapolis: Jones Strain-Counterstrain Inc.; 1995.
Haman JL. An osteopathic approach to treating chondromalacia-patellae with counterstrain manipulation. American Association Osteopathic Journal; 1994:26e7.
Howell JN, Cabell KS, Chila AG, Eland DC. Stretch reflex and Hoffman reflex responses to osteopathic manipulative treatment in subjects with Achilles tendonitis. Journal of the American Osteopathic Association 2006;106:537e45.
Huijing PA, Baar G. Myofascial force transmission via extramuscular pathways occurs between antagonistic muscles. Cells Tissues Organs 2008;188(4):400e14.
Ibáñez-García J, Alburquerque-Sendín F, Rodríguez-Blanco C, Girao D, Atienza- Meseguer A, Planella-Abella S, et al. Changes in masseter muscle trigger points following strain-counterstrain or neuro-muscular technique. Journal of Bodywork and Movement Therapies 2009;13:2e10.
Jacobson EC, Lockwood HJ, Dickey JL, Kuchera WL. Shoulder pain and repetition strain injury to the supraspinatus muscle: etiology and manipulative treatment. Journal of the American Osteopathic Association 1990;89(8):1037e40.
Johnson SM, Kurtz ME. Osteopathic manipulative treatment techniques preferred by contemporary osteopathic physicians. Journal of the American Osteopathic Association 2003;103(5):219e24.
Jones LH. Foot treatment without hand trauma. Journal of the American Osteo- pathic Association 1973;72:481e9.
Jones LH. Spontaneous release by positioning. Doctor of Osteopathy 1964;4:109e16. Jones LH. Strain-counterstrain. Indianapolis: Jones Strain-Counterstrain Inc; 1995. Kopf M, Baumann H, Freer G, Freudenberg M, Lamers M, Kishimoto T, et al. Impaired
immune and acute-phase responses in interleukin-6-deficient mice. Nature
1994;368:339e42.
Korr IM. Proprioceptors and somatic dysfunction. Journal of the American Osteo-
pathic Association 1975;74:638e50.
Kreulen M, Smeulders M, Hage JJ, Huijing PA. Biomechanical effects of dissecting
flexor carpi ulnaris. Journal of Bone and Joint Surgery [Br] 2003;85(6):856e9. Krogsgaard MR, Dyhre-Poulsen P, Fischer-Rasmussen T. Cruciate ligament reflexes.
Journal of Electromyography and Kinesiology 2002;12:177e82.
Kusunose RS. Strain and counterstrain in the manual medicine armamentarium. In:
Strain-counterstrain. Indianapolis: Jones Strain-Counterstrain Inc.; 1995.
Lewis C, Flynn T. The use of strain counterstrain in the treatment of patients with
low back pain. Journal of Manual & Manipulative Therapy 2001;9(2):92e8. Lewis C, Khan A, Souvlis T, Sterling M. A randomised controlled study examining the short-term effects of strain-counterstrain treatment on quantitative sensory measures at digitally tender points in the low back. Manual Therapy 2010a;15:
536e41.
Lewis C, Souvlis T, Sterling M. Sensory characteristics of tender points in the lower
back. Manual Therapy 2010b;15:451e6.
Lewis C, Sterling M, Souvlis T. Digitally tender points: their significance in physio-
therapy. Physical Therapy Reviews 2008;13(3):188e96.
Licciardone JC. The unique role of osteopathic physicians in treating patients with
low back pain. Journal of the American Osteopathic Association 2004;104(11):
S13e8.
McNair PJ, Marshall RN, Maguire K. Swelling of the knee joint: effects on quadriceps
muscle strength. Archives of Physical Medicine and Rehabilitation 1996;77(9):
896e9.
McPartland JM, Goodridge JP. Counterstrain and traditional osteopathic examina-
tion of the cervical spine compared. Journal of Bodywork and Movement
Therapies 1997;1(3):173e8.
Meltzer KR, Standley PR. Modeled repetitive motion strain and indirect osteopathic
manipulative techniques on regulation of human fibroblast proliferation and interleukin secretion. Journal of the American Osteopathic Association 2007; 107:527e36.
Mense S, Simons DG. Muscle pain e understanding its nature, diagnosis, and treatment. Philadelphia: Lippincott; 2001.
Meseguer A, Fernandez-de-las-Penas C, Navarro-Poza JL, Rodriguez-Blanco C, Bosca Gandia JJ. Immediate effects of the strain-counterstrain technique in local pain evoked by tender points in the upper trapezius muscle. Clinical Chiropractic 2006;9:112e8.
C.K. Wong / Manual Therapy 17 (2012) 2e8 7
8 C.K. Wong / Manual Therapy 17 (2012) 2e8
Nagrale AVGP, Joshi A, Ramteke G. The efficacy of an integrated neuromuscular inhibition technique on upper trapezius trigger points in subjects with non- specific neck pain: a randomized controlled trial. Journal of Manual & Manip- ulative Therapy 2010;18:37e43.
Pedowitz RN. Use of osteopathic manipulative treatment for iliotibial band friction syndrome. Journal of the American Osteopathic Association 2005;105(12):563e7. Perreault A, Kelln B, Hertel J, Pugh K, Saliba S. Short-term effects of strain coun- terstrain in reducing pain in upper trapezius tender points. Athletic Training
and Sports Health Care 2009;1(5):214e21.
Radjieski JM, Lumley MA, Cantieri MS. Effect of osteopathic manipulative treatment
on length of stay for pancreatitis: a randomized pilot study. Journal of the
American Osteopathic Association 1998;98:264e72.
Ramirez MA, Haman JL, Worth L. Low back pain: diagnosis by six newly discovered
sacral tender points and treatment with counterstrain. Journal of the American
Osteopathic Association 1989;89:905e13.
Rathbun J, Macnab I. Microvascular pattern at the rotator cuff. Journal of Bone and
Joint Surgery [Am] 1970;52:540e53.
Schwartz HR. The use of counterstrain in an acutely ill in-hospital population.
Journal of the American Osteopathic Association 1986;86:433e42.
Simons DG, Travell JG, Simons LS. Myofascial pain and dysfunction: the trigger
point manual, vol. 1. Baltimore: Williams & Wilkins; 1999. 70.
Solomonow M, Lewis J. Reflex from the ankle ligaments of the feline. Journal of
Electromyography and Kinesiology 2002;12:193e8.
Solomonow M. Ligaments: a source of musculoskeletal disorders. Journal of Bodywork and Movement Therapies 2009;13:136e54.
Sterling M, Jull G, Vincenzino B, Kenardy J, Darnell R. Development of motor system dysfunction following whiplash injury. Pain 2003;103:65e73.
Walko EJ, Janouschek C. Effects of osteopathic manipulative treatment in patients with cervicothoracic pain: pilot study in thermography. Journal of the American Osteopathic Association 1994;94:135e41.
Wong CK, Harris V, Gidali A. Deformity or dysfunction? Osteopathic manipulation of the idiopathic cavus foot: a clinical suggestion. North American Journal of Sports Physical Therapy 2010;5(1):27e32.
Wong CK, Moskowitz N, Fabillar R. Effect of strain counterstrain on for forearm strength compared to placebo positioning. International Journal of Osteopathic Medicine, in press. Available online 21 January 2011: doi:10.1016/ j.ijosm.2010.11.004770.
Wong CK, Schauer CS. Reliability, validity, and effectiveness of strain counter- strain techniques. Journal of Manual and Manipulative Therapy 2004;12(2): 107e12.
Wong CK, Schauer-Alvarez CS. The effect of strain counterstrain on pain and strength. Journal of Manual and Manipulative Therapy 2004;12(4):215e24. Wynn MW, Burns JM, Eland DC, Conatser RR, Howell JN. Effect of counterstrain on
stretch reflexes, Hoffman reflexes and clinical outcomes in subjects with plantar fasciitis. Journal of the American Osteopathic Association 2006;106: 547e56.